Faculty Research
My research interests are in the broad field of organic synthesis. Projects in my group range from developing new synthetic methods and asymmetric reactions to the total synthesis of natural products possessing interesting structural features and biological properties. Our work often involves epoxide opening reactions and requires detailed analysis of NMR spectra to characterize synthetic intermediates.
Research Projects
Total Synthesis of Guaipyridine Alkaloid Natural Products
The guaipyridines are a small family of alkaloids isolated from various Asian plants. Cananodine is isolated from the fruit of Cananga odorata (ylang-ylang), a tree whose bark, leaves, and oil have been used in the folk medicine of SE Asia. Cananodine exhibits encouraging activity against two types of hepatollular carcinoma. Cananodine was also detected recently as a minor component of cypriol oil. The rupestines are isolated from the dried leaves and flowers of Artemesia rupestris (rock wormwood), a plant native to SW China. This plant has also been used in traditional Chinese medicine for a variety of maladies, including to protect the liver. Our strategy for approaching the guaipyridine skeleton involves alkylation at the picolyl position and Pd-catalyzed coupling to the pyridine ring to form the seven-membered carbocycle.
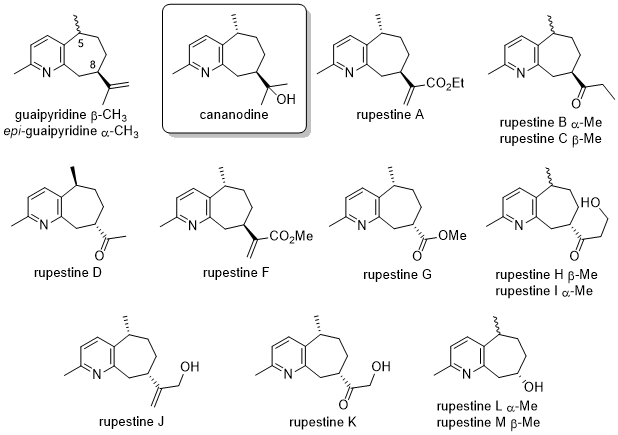
References:
Hsieh, T.-J.; Chang, F.-R.; Chia, Y.-C.; Chen, C.-Y.; Chiu, H.-F.; Wu, Y.-C. Cytotoxic Constituents of the Fruits of Cananga odorata. J. Nat. Prod. 2001, 64, 616-9.
Su, A.; Wu, H.-K.; He, H.; Slukhan, U.; Aisa, H. A. New Guaipyridine Sesquiterpene Alkaloids from Artemesia rupestris L. Helv. Chim. Acta 2010, 93, 33 – 38.
He, F.; Nugroho, A. E.; Wong, C. P.; Hirasawa, Y.; Shirota, O.; Morita, H.; Aisa, H. A. Rupestines F-M, New Guaipyridine Sesquiterpene Alkaloids from Artemesia rupestris. Chem. Pharm Bull. 2012, 60, 213-218.
Craig, D.; Henry, G. D.; Total Synthesis of Cytotoxic Guaipyridine Sesquiterpene Alkaloid (+)-Cananodine. Eur. J. Org. Chem. 2006, 3558-3561.
Shelton, P.; Ligon, T. J.; Dell, J. M.; Yarbrough, L.; Vyvyan, J. R. Synthesis of cananodine by intramolecular epoxide opening. Tetrahedron Lett. 2017, 58, 3478-3481. DOI: 10.1016/j.tetlet.2017.07.080.
Starchman, E. S.; Marshall, M. S.; Vyvyan, J. R. Synthesis of (±)-rupestines B and C by intramolecular Mizoroki-Heck reaction. Tetrahedron Lett. 2020, 61, 151837. https://doi.org/10.1016/j.tetlet.2020.151837
Shelton, P. M. M.; Grosslight, S. M.; Mulligan, B. J.; Spargo, H. V.; Saad, S. S.; Vyvyan, J. R. Synthesis of guaipyridine alkaloids (±)-cananodine and (±)-rupestines D and G using an intramolecular Mizoroki-Heck reaction. Tetrahedron 2020, 76, in press. https://doi.org/10.1016/j.tet.2020.131500.
New Gold-Catalyzed Reactions
We have found that gold complexes catalyze the conversion of unsaturated benzyl esters to lactones. We are currently exploring the scope and stereoselectivity of this transformation.

